Contents
- Analysis of variance (ANOVA)
- ONE-WAY ANOVA
- Non-parametric version of the Analysis of Variance
- hovPlot
- Contrasts in ANOVA
- Factorial ANOVA
- Pseudoreplication: Nested designs and split plots
- Split-plot experiments
- Effect sizes in ANOVA: aov or lm?
- ANOVA for repeated measures
Anova or analysis of variance makes basically the same assumptions as a t-test (normally distributed responses), but allows for more than two groups. More precisely, the measured response (i.e. the dependent variable) can be influence by several categorical variables that could also interact. Here a simple example for testing whether weight depends on group, where group is a variable that codes for three different options control, treatment1 and treatment2:
library(knitr)
## Warning: package 'knitr' was built under R version 3.0.3
opts_knit$set(global.par=TRUE)
opts_chunk$set(cache.extra = rand_seed,fig.align='center')
set.seed(13)
aovresult <- aov(extra~group, data = sleep)
summary(aovresult)
## Df Sum Sq Mean Sq F value Pr(>F)
## group 1 12.5 12.5 3.46 0.079 .
## Residuals 18 64.9 3.6
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
We find a p-value of 0.0159, which is significant at an $\alpha$ level of 0.05. Note that in this case, we don’t get any parameter estimates. If you want those, there are two options for the ANOVA: \begin{itemize} \item Either you apply what is called post-hoc testing, which means that you test for differences (e.g. with a t-test) between the subgroups, i.e. control vs. treatment1, treatment1 vs. treatment2, etc. \item Or you switch to a regression, which is described in the next chapter \end{itemize} If you do post-hoc testing, you are doing multiple tests on the same data. This is a problem - the idea of the p-value is that you calculate the probability of seeing the data under ONE null hypothesis. If you do this, you will get at most 5\% error at an $\alpha$ level of 0.05. \marginnote{When doing multiple tests on the same data, we need to correct the p-values for multiple testing.} However, if we do multiple tests, we are testing multiple null hypotheses, and there are more options for the test statistics to get significant just by chance. Hence, we need to correct the p-values for multiple testing. There are a number of options to do so, google is your friend.
ONE-WAY ANOVA
ANOVA is a parametric method appropriate to compare means of two or more INDEPENDENT populations by using variances. Do not confuse with ANCOVA, a model which blends ANOVA and regression
Model validation, assumptions: 1) Random sampling, or at the very least, random assignment to groups. Independence of scores on the response variable. What we get from one subject should be in no way influenced by what we get from any of the others. 2) Variation of observations around the regression line (the residual standard error) is CONSTANT (homoscedasticity) 3) Y values (or the errors) are INDEPENDENT (independence) 4) For a given X value, Y values (or the errors) are NORMALLY DISTRIBUTED (normality)
Example, clay’s yield depending on the soil
results <- read.delim("Z:/GitHub/RMarkdowns - Stat with R/6_ANOVA/yields.txt")
results
## sand clay loam
## 1 6 17 13
## 2 10 15 16
## 3 8 3 9
## 4 6 11 12
## 5 14 14 15
## 6 17 12 16
## 7 9 12 17
## 8 11 8 13
## 9 7 10 18
## 10 11 13 14
attach(results)
names(results)
## [1] "sand" "clay" "loam"
#the datset is horizonetal, first we will flip it to vertical
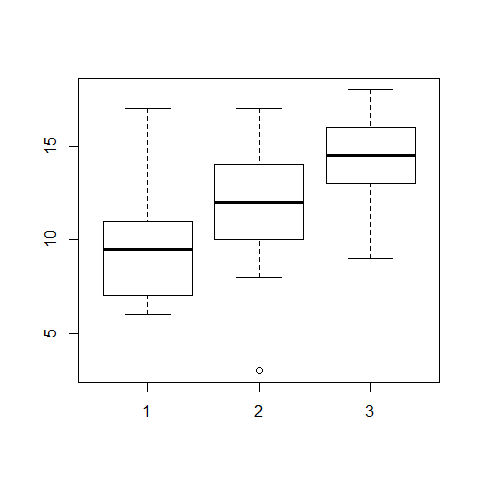
sapply(list(sand,clay,loam),mean) #for horizontal datasets
## [1] 9.9 11.5 14.3
boxplot(sand,clay,loam)
#to flip the dataset
frame <- stack(results)
names(frame) <- c("yield","soil")
detach(results)
attach(frame)
head(frame)
## yield soil
## 1 6 sand
## 2 10 sand
## 3 8 sand
## 4 6 sand
## 5 14 sand
## 6 17 sand
#now, the dataset is vertical
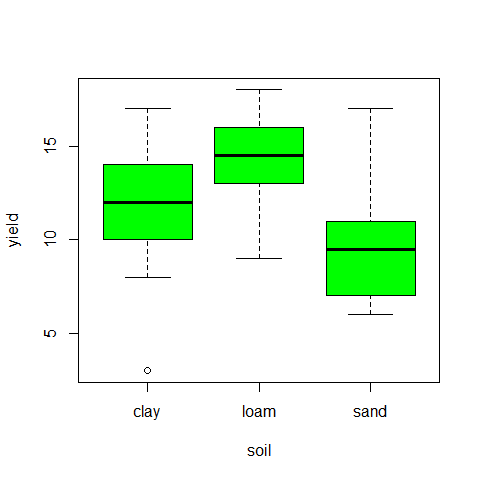
tapply(yield,soil,var)
## clay loam sand
## 15.389 7.122 12.544
plot(yield~soil,col="green")
#test for homoscedasticity, one of the model assumptions
fligner.test(yield~soil) # Fligner-Killeen test of homogeneity of variances
##
## Fligner-Killeen test of homogeneity of variances
##
## data: yield by soil
## Fligner-Killeen:med chi-squared = 0.3651, df = 2, p-value = 0.8332
bartlett.test(yield~soil) #Bartlett.test
##
## Bartlett test of homogeneity of variances
##
## data: yield by soil
## Bartlett's K-squared = 1.276, df = 2, p-value = 0.5283
#Once tested the assumptions, we can compute the ANOVA
model=aov(yield~soil)
summary(model)
## Df Sum Sq Mean Sq F value Pr(>F)
## soil 2 99.2 49.6 4.24 0.025 *
## Residuals 27 315.5 11.7
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
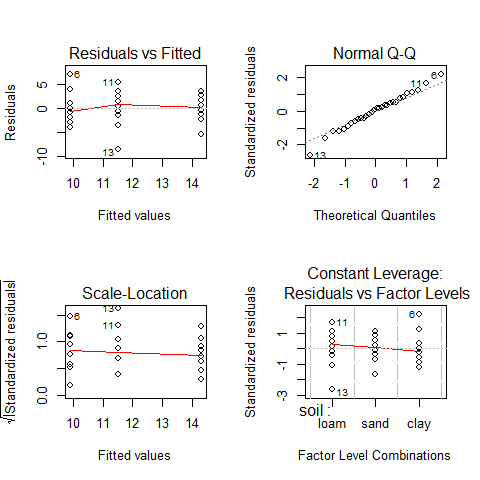
par(mfrow=c(2,2))
plot(aov(yield~soil))
par(mfrow=c(1,1))
#we can see that the assumptions are met
detach(frame)
ONE-WAY ANOVA example: Load weight loss depending on 4 different diets
diets <- read.delim("Z:/GitHub/RMarkdowns - Stat with R/6_ANOVA/DietWeigthLoss.txt")
head(diets)
## WeightLoss Diet
## 1 9.9 A
## 2 9.6 A
## 3 8.0 A
## 4 4.9 A
## 5 10.2 A
## 6 9.0 A
summary(diets)
## WeightLoss Diet
## Min. : 3.80 A:15
## 1st Qu.: 8.97 B:15
## Median :10.20 C:15
## Mean :10.19 D:15
## 3rd Qu.:11.90
## Max. :15.10
attach(diets)
class(WeightLoss) #our response variable
## [1] "numeric"
class(Diet) # our predictor variable (categorical)
## [1] "factor"
levels(Diet)
## [1] "A" "B" "C" "D"
length(WeightLoss)
## [1] 60
boxplot(WeightLoss~Diet, ylab="Weight loss (kg)", xlab="Diet ",col=c(0,2,3,4))
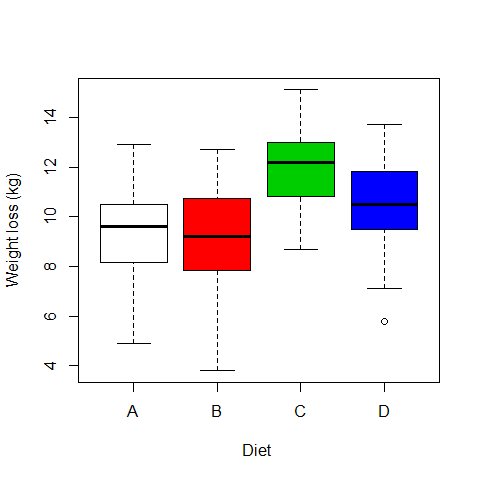
# Ho = mean equal for all diets
model1= aov(WeightLoss~Diet)
summary(model1)
## Df Sum Sq Mean Sq F value Pr(>F)
## Diet 3 97.3 32.4 6.12 0.0011 **
## Residuals 56 297.0 5.3
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
attributes(model1)
## $names
## [1] "coefficients" "residuals" "effects" "rank"
## [5] "fitted.values" "assign" "qr" "df.residual"
## [9] "contrasts" "xlevels" "call" "terms"
## [13] "model"
##
## $class
## [1] "aov" "lm"
model1$coefficients #we subset the coefficients from the model's attributes
## (Intercept) DietB DietC DietD
## 9.1800 -0.2733 2.9333 1.3600
mean(WeightLoss[Diet=="A"]) #coefficients have been estimated with respect to Diet A
## [1] 9.18
Multiple comparisons
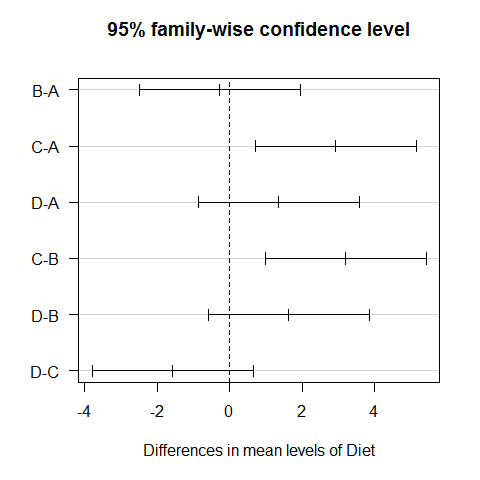
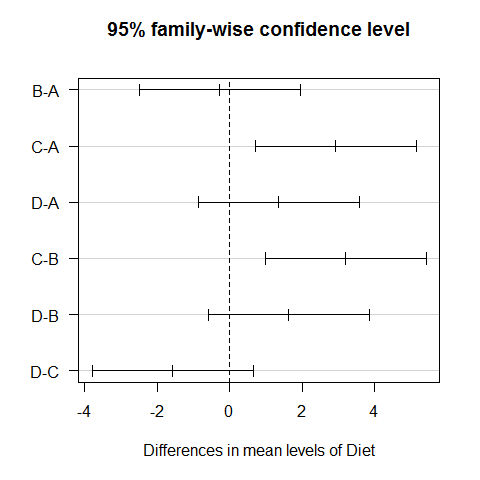
TukeyHSD(model1) #all possible combinations between the different diets
## Tukey multiple comparisons of means
## 95% family-wise confidence level
##
## Fit: aov(formula = WeightLoss ~ Diet)
##
## $Diet
## diff lwr upr p adj
## B-A -0.2733 -2.4999 1.9533 0.9880
## C-A 2.9333 0.7067 5.1599 0.0051
## D-A 1.3600 -0.8666 3.5866 0.3774
## C-B 3.2067 0.9801 5.4333 0.0019
## D-B 1.6333 -0.5933 3.8599 0.2224
## D-C -1.5733 -3.7999 0.6533 0.2521
plot(TukeyHSD(model1), las=1) #graphic visualization
ANOVA’s assumptions
par(mfrow=c(2,2))
plot(model1)
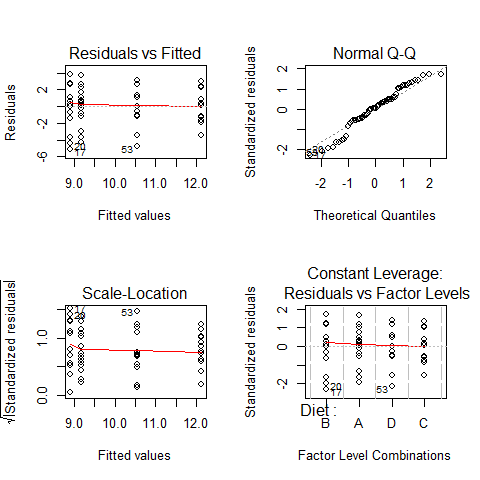
par(mfrow=c(1,1))
qqnorm(model1$residuals);qqline(model1$residuals)
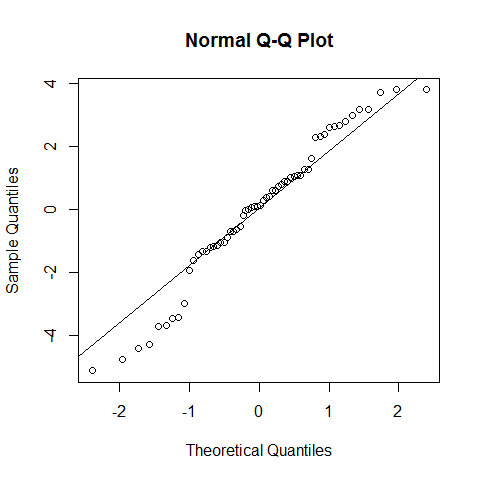
shapiro.test(model1$residuals) # we cannot reject the null hypothesis, but with poor confidence. However, as discussed for the linear regression, the assumption of normality is not that strict.
##
## Shapiro-Wilk normality test
##
## data: model1$residuals
## W = 0.9646, p-value = 0.07918
If our data is no normally distributed: The one-way ANOVA is considered a robust test against the normality assumption. This means that it tolerates violations to its normality assumption rather well. Regarding the normality of the group data, the one-way ANOVA can tolerate data that is non-normal . However, platykurtosis can have a profound effect when the group sizes are small. (Kurtosis.jpg)
There are two options: 1) Transform the data using various algorithms so that the shape of the distributions become normally distributed 2) Choose the non-parametric Kruskal-Wallis H Test which does not require the assumption of normality.
par(mfrow=c(1,2))
hist(model1$residuals,freq=F,ylim=c(0,0.4),breaks=10);lines(density(model1$residuals))
hist(WeightLoss,freq=F,ylim=c(0,0.4),breaks=10);lines(density(WeightLoss))
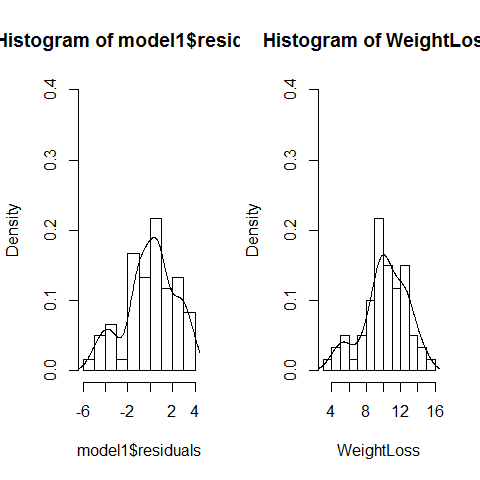
par(mfrow=c(1,1))
#Platykurtosis was not used here, the sample size is not small neither.
It is important to remember, that the homogeneity of variances is more important than the normality of residuals
Other option to run a parametric one-way ANOVA with correction for non-equal variances, limited to very simple ANOVAs:
data(InsectSprays)
attach(InsectSprays)
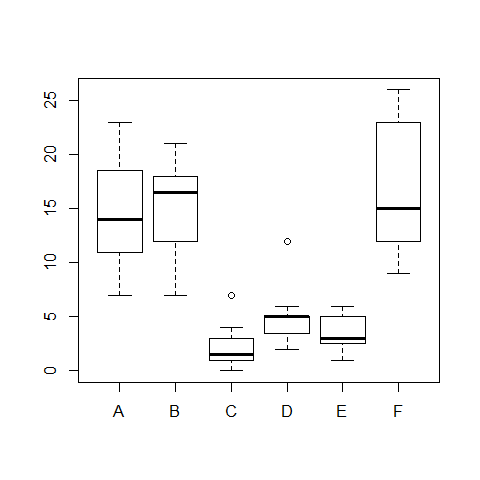
fligner.test(count ~ spray) # variances are not equal
##
## Fligner-Killeen test of homogeneity of variances
##
## data: count by spray
## Fligner-Killeen:med chi-squared = 14.48, df = 5, p-value = 0.01282
boxplot(count ~ spray)
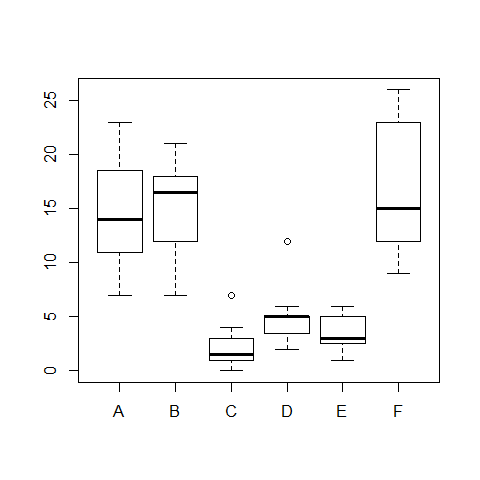
model3=oneway.test(count ~ spray) #this is not assuming equal variances
model3
##
## One-way analysis of means (not assuming equal variances)
##
## data: count and spray
## F = 36.07, num df = 5.00, denom df = 30.04, p-value = 7.999e-12
detach(InsectSprays)
More complex example: Serum_iron(microg/dL) dosis in 120 patients
iron <- read.delim("Z:/GitHub/RMarkdowns - Stat with R/6_ANOVA/hospital_ANOVA.txt")
head(iron)
## Serum_iron treatment
## 1 69.78 DrugA
## 2 91.48 DrugA
## 3 67.29 DrugA
## 4 90.71 DrugA
## 5 72.02 DrugA
## 6 71.35 DrugA
dim(iron) #dimensions of the data
## [1] 120 2
attach(iron)
levels(treatment)
## [1] "DrugA" "DrugB" "DrugC" "placebo"
Running a parametric ANOVA
boxplot(Serum_iron~treatment)
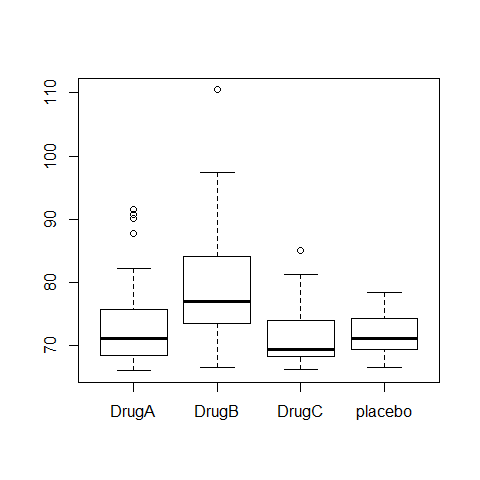
ANOVA1=aov(Serum_iron~treatment)
summary(ANOVA1)
## Df Sum Sq Mean Sq F value Pr(>F)
## treatment 3 1385 462 9.81 8.1e-06 ***
## Residuals 116 5458 47
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
TukeyHSD(ANOVA1)
## Tukey multiple comparisons of means
## 95% family-wise confidence level
##
## Fit: aov(formula = Serum_iron ~ treatment)
##
## $treatment
## diff lwr upr p adj
## DrugB-DrugA 5.5409 0.9244 10.158 0.0118
## DrugC-DrugA -2.6640 -7.2806 1.953 0.4383
## placebo-DrugA -2.8916 -7.5081 1.725 0.3645
## DrugC-DrugB -8.2050 -12.8215 -3.588 0.0001
## placebo-DrugB -8.4325 -13.0491 -3.816 0.0000
## placebo-DrugC -0.2275 -4.8441 4.389 0.9992
op=par(mar=c(4,8,4,4))
plot(TukeyHSD(ANOVA1),las=1)
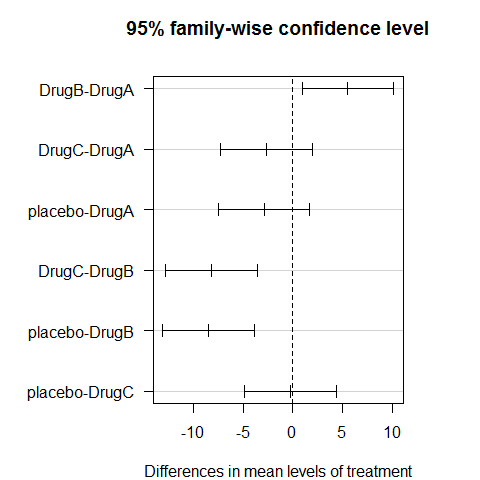
par(op)
op=par(mfrow=c(2,2))
plot(ANOVA1)
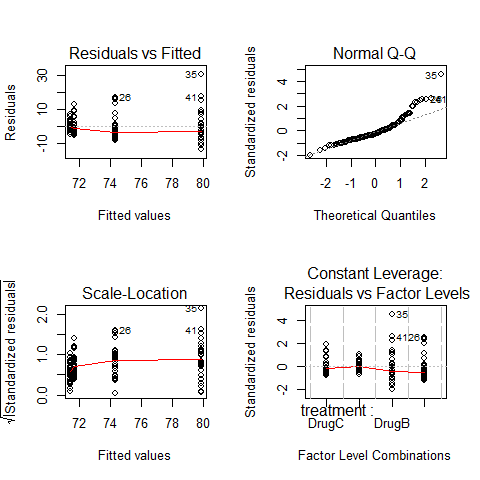
par(op)
par(mfrow=c(1,1))
fligner.test(Serum_iron~treatment)
##
## Fligner-Killeen test of homogeneity of variances
##
## data: Serum_iron by treatment
## Fligner-Killeen:med chi-squared = 9.948, df = 3, p-value = 0.01902
bartlett.test(Serum_iron~treatment, iron)
##
## Bartlett test of homogeneity of variances
##
## data: Serum_iron by treatment
## Bartlett's K-squared = 35.6, df = 3, p-value = 9.1e-08
#increasing spred in residuals, different spread in residuals depending on drugs
boxplot(ANOVA1$residuals~treatment)
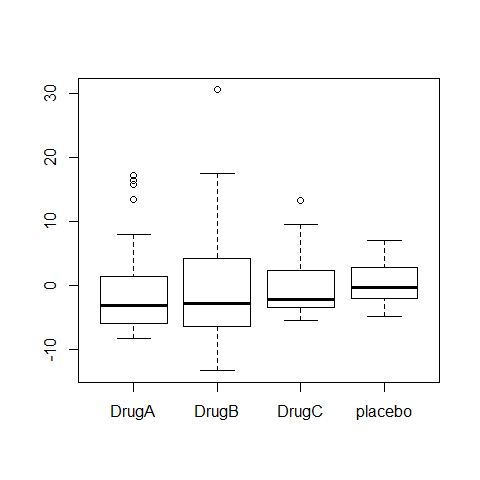
# There is an independence problem, we need more information: are patients all from the same hospital? what about sex and age of patient?
Non-parametric version of the Analysis of Variance
KRUSKAS WALLIS 1-WAY Analysis of Variance is a non-parametric equivalent of ANOVA for independent samples.
model2=kruskal.test(WeightLoss~Diet)
library(pgirmess)
## Warning: package 'pgirmess' was built under R version 3.0.3
kruskalmc(WeightLoss~Diet)
## Multiple comparison test after Kruskal-Wallis
## p.value: 0.05
## Comparisons
## obs.dif critical.dif difference
## A-B 1.167 16.82 FALSE
## A-C 21.167 16.82 TRUE
## A-D 9.733 16.82 FALSE
## B-C 22.333 16.82 TRUE
## B-D 10.900 16.82 FALSE
## C-D 11.433 16.82 FALSE
plot(TukeyHSD(model1), las=1) #compare with results of Tukey plot (line 91)
detach(diets)
However, the Kruskal-Wallis test should not be regarded as an universal solution, it is primarily intended to solve normality problems. It performs better when the distributions have all the same shape, and when there is homogeneity of variance.
kruskal.test(Serum_iron~treatment)
##
## Kruskal-Wallis rank sum test
##
## data: Serum_iron by treatment
## Kruskal-Wallis chi-squared = 24.04, df = 3, p-value = 2.449e-05
library(pgirmess)
kruskalmc(Serum_iron~treatment) #compare with the results of Tukey plot, they are the same
## Multiple comparison test after Kruskal-Wallis
## p.value: 0.05
## Comparisons
## obs.dif critical.dif difference
## DrugA-DrugB 29.033 23.7 TRUE
## DrugA-DrugC 10.767 23.7 FALSE
## DrugA-placebo 6.667 23.7 FALSE
## DrugB-DrugC 39.800 23.7 TRUE
## DrugB-placebo 35.700 23.7 TRUE
## DrugC-placebo 4.100 23.7 FALSE
detach(iron)
hovPlot
The hovPlot( ) function in the HH package provides a graphic test of homogeneity of variances based on Brown-Forsyth. Oneway analysis of variance makes the assumption that the variances of the groups are equal.
Example 1 (clearly not homogeneous variances)
# Homogeneity of Variance Plot
library(HH)
## Warning: package 'HH' was built under R version 3.0.3
## Loading required package: lattice
## Warning: package 'lattice' was built under R version 3.0.3
## Loading required package: grid
## Loading required package: latticeExtra
## Warning: package 'latticeExtra' was built under R version 3.0.3
## Loading required package: RColorBrewer
## Loading required package: multcomp
## Warning: package 'multcomp' was built under R version 3.0.3
## Loading required package: mvtnorm
## Warning: package 'mvtnorm' was built under R version 3.0.3
## Loading required package: survival
## Loading required package: splines
## Loading required package: TH.data
## Warning: package 'TH.data' was built under R version 3.0.3
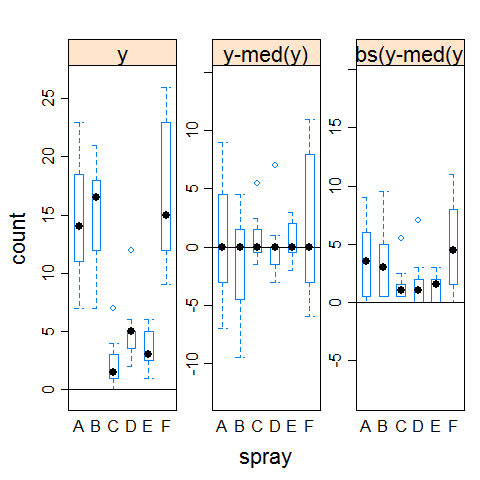
boxplot(count ~ spray,data = InsectSprays)
hov(count ~ spray, data = InsectSprays)
##
## hov: Brown-Forsyth
##
## data: count
## F = 3.821, df:spray = 5, df:Residuals = 66, p-value = 0.004223
## alternative hypothesis: variances are not identical
hovPlot(count ~ spray, data = InsectSprays)
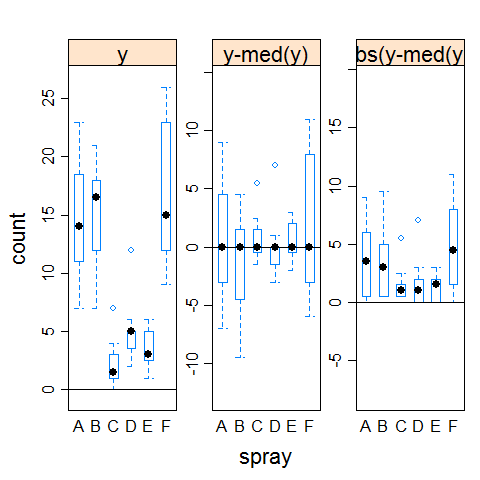
Example 2 (homogeneous variances)
data(turkey)
head(turkey)
## diet wt.gain
## 1 control 4.1
## 2 control 3.3
## 3 control 3.1
## 4 control 4.2
## 5 control 3.6
## 6 control 4.4
boxplot(wt.gain ~ diet, data=turkey)
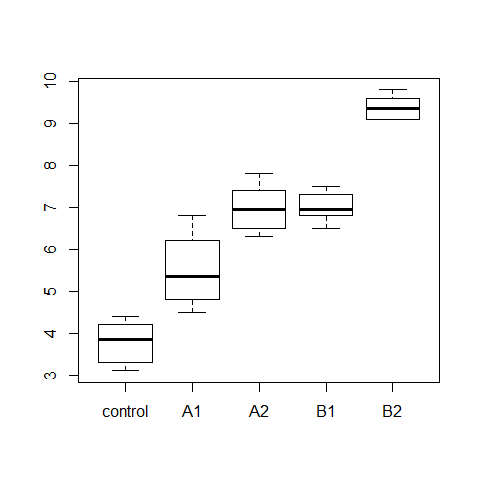
model3=aov(wt.gain ~ diet, data=turkey)
summary(model3)
## Df Sum Sq Mean Sq F value Pr(>F)
## diet 4 103.0 25.76 81.7 5.6e-14 ***
## Residuals 25 7.9 0.32
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
par(mfrow=c(2,2))
plot(model3)
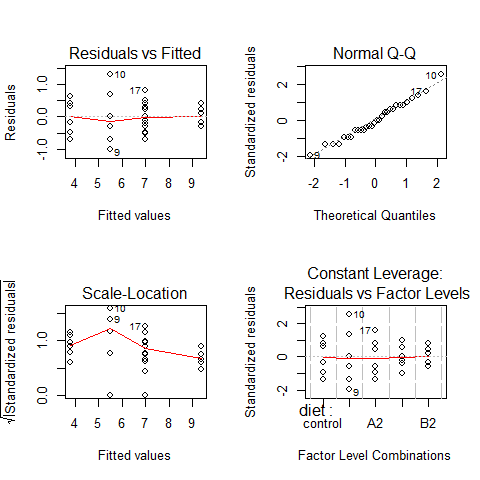
par(mfrow=c(1,1))
hov(wt.gain ~ diet, data=turkey)
##
## hov: Brown-Forsyth
##
## data: wt.gain
## F = 2.226, df:diet = 4, df:Residuals = 25, p-value = 0.09506
## alternative hypothesis: variances are not identical
hovPlot(wt.gain ~ diet, data=turkey)
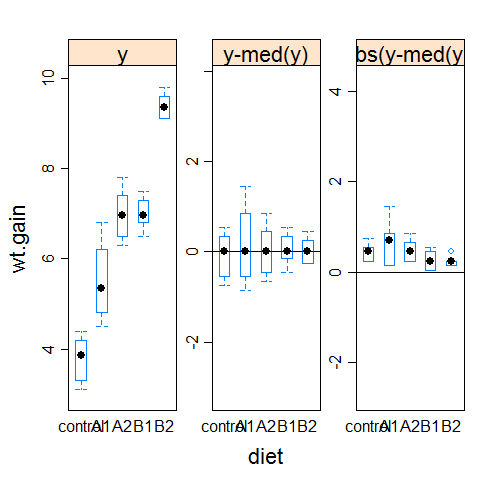
#compare it with the example1 plot
hovPlot(count ~ spray, data = InsectSprays)
Contrasts in ANOVA
1) Treatment contrasts in ANOVA using summary.lm instead of summary()
When the design consists of a control condition and several treatments, or when there is a distinct baseline condition to which the other groups can be logically compared, then treatment constrasts offers a mechanism to visualize the treatment effects.
attach(PlantGrowth)
summary(PlantGrowth)
## weight group
## Min. :3.59 ctrl:10
## 1st Qu.:4.55 trt1:10
## Median :5.16 trt2:10
## Mean :5.07
## 3rd Qu.:5.53
## Max. :6.31
boxplot(weight ~ group)
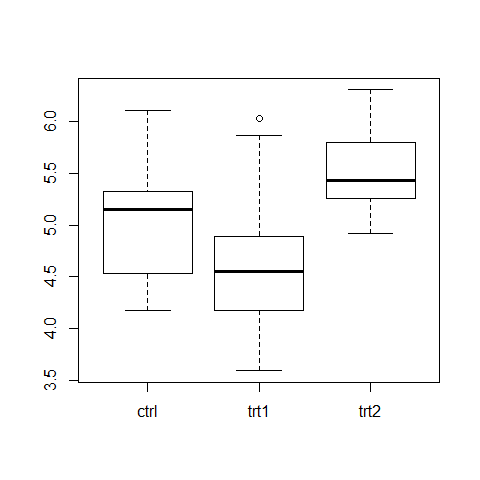
bartlett.test(weight ~ group)
##
## Bartlett test of homogeneity of variances
##
## data: weight by group
## Bartlett's K-squared = 2.879, df = 2, p-value = 0.2371
results = aov(weight ~ group)
summary.aov(results) #classical approach
## Df Sum Sq Mean Sq F value Pr(>F)
## group 2 3.77 1.883 4.85 0.016 *
## Residuals 27 10.49 0.389
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
TukeyHSD(results) #classical approach
## Tukey multiple comparisons of means
## 95% family-wise confidence level
##
## Fit: aov(formula = weight ~ group)
##
## $group
## diff lwr upr p adj
## trt1-ctrl -0.371 -1.0622 0.3202 0.3909
## trt2-ctrl 0.494 -0.1972 1.1852 0.1980
## trt2-trt1 0.865 0.1738 1.5562 0.0120
plot(TukeyHSD(results))
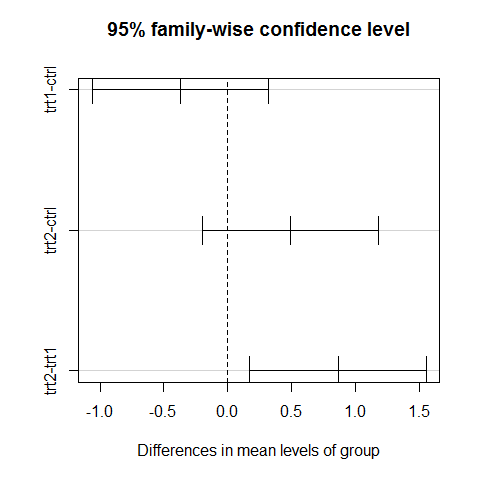
summary.lm(results)
##
## Call:
## aov(formula = weight ~ group)
##
## Residuals:
## Min 1Q Median 3Q Max
## -1.071 -0.418 -0.006 0.263 1.369
##
## Coefficients:
## Estimate Std. Error t value Pr(>|t|)
## (Intercept) 5.032 0.197 25.53 <2e-16 ***
## grouptrt1 -0.371 0.279 -1.33 0.194
## grouptrt2 0.494 0.279 1.77 0.088 .
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
##
## Residual standard error: 0.623 on 27 degrees of freedom
## Multiple R-squared: 0.264, Adjusted R-squared: 0.21
## F-statistic: 4.85 on 2 and 27 DF, p-value: 0.0159
detach(PlantGrowth)
The treatment contrasts are in the Coefficients table. The estimated coefficient for the Intercept is the mean of the baseline or control group. But if the final aim is to do all the possible comparisons anyway, the better solution is to use the Tukey’s HSD test, which controls the experimentwise error rate.
2) More on contrasts - controlling the significance in multiple comparisons
The following example concerns the yield of fungus gathered from 16 different habitats:
data <- read.delim("Z:/GitHub/RMarkdowns - Stat with R/6_ANOVA/Fungi.txt")
attach(data)
First we establish whether there is any variation in fungus yield to explain:
model <- aov(Fugus.yield~Habitat)
summary(model)
## Df Sum Sq Mean Sq F value Pr(>F)
## Habitat 15 7527 502 72.1 <2e-16 ***
## Residuals 144 1002 7
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
There is (p < 0.000 001). But this is not too interesting, because it just shows that some habitats produce more fungus than others. We are interested in which habitats produce significantly more fungi than others. Multiple comparisons can be an issue since there are 16 habitats (16 Ã 15)/2 = 120 possible pairwise comparisons.
There are two options:
TukeyHSD(model)
## Tukey multiple comparisons of means
## 95% family-wise confidence level
##
## Fit: aov(formula = Fugus.yield ~ Habitat)
##
## $Habitat
## diff lwr upr p adj
## Ash-Alder 3.53293 -0.58081 7.6467 0.1844
## Aspen-Alder 12.78574 8.67201 16.8995 0.0000
## Beech-Alder 12.32365 8.20992 16.4374 0.0000
## Birch-Alder 14.11348 9.99974 18.2272 0.0000
## Cherry-Alder 10.29509 6.18135 14.4088 0.0000
## Chestnut-Alder 12.24108 8.12734 16.3548 0.0000
## Holmoak-Alder -1.44361 -5.55734 2.6701 0.9976
## Hornbeam-Alder 10.60271 6.48897 14.7164 0.0000
## Lime-Alder 19.19458 15.08084 23.3083 0.0000
## Oak-Alder 20.29457 16.18084 24.4083 0.0000
## Pine-Alder 14.34085 10.22711 18.4546 0.0000
## Rowan-Alder 6.29495 2.18121 10.4087 0.0000
## Spruce-Alder -2.15119 -6.26493 1.9625 0.9037
## Sycamore-Alder 2.80900 -1.30474 6.9227 0.5645
## Willow-Alder 2.77635 -1.33739 6.8901 0.5849
## Aspen-Ash 9.25282 5.13908 13.3666 0.0000
## Beech-Ash 8.79073 4.67699 12.9045 0.0000
## Birch-Ash 10.58055 6.46682 14.6943 0.0000
## Cherry-Ash 6.76216 2.64842 10.8759 0.0000
## Chestnut-Ash 8.70815 4.59441 12.8219 0.0000
## Holmoak-Ash -4.97653 -9.09027 -0.8628 0.0043
## Hornbeam-Ash 7.06978 2.95605 11.1835 0.0000
## Lime-Ash 15.66165 11.54792 19.7754 0.0000
## Oak-Ash 16.76165 12.64791 20.8754 0.0000
## Pine-Ash 10.80792 6.69418 14.9217 0.0000
## Rowan-Ash 2.76202 -1.35171 6.8758 0.5938
## Spruce-Ash -5.68412 -9.79786 -1.5704 0.0004
## Sycamore-Ash -0.72393 -4.83766 3.3898 1.0000
## Willow-Ash -0.75658 -4.87031 3.3572 1.0000
## Beech-Aspen -0.46209 -4.57583 3.6516 1.0000
## Birch-Aspen 1.32774 -2.78600 5.4415 0.9990
## Cherry-Aspen -2.49066 -6.60439 1.6231 0.7546
## Chestnut-Aspen -0.54467 -4.65840 3.5691 1.0000
## Holmoak-Aspen -14.22935 -18.34309 -10.1156 0.0000
## Hornbeam-Aspen -2.18303 -6.29677 1.9307 0.8930
## Lime-Aspen 6.40884 2.29510 10.5226 0.0000
## Oak-Aspen 7.50883 3.39509 11.6226 0.0000
## Pine-Aspen 1.55510 -2.55863 5.6688 0.9946
## Rowan-Aspen -6.49079 -10.60453 -2.3771 0.0000
## Spruce-Aspen -14.93694 -19.05068 -10.8232 0.0000
## Sycamore-Aspen -9.97674 -14.09048 -5.8630 0.0000
## Willow-Aspen -10.00939 -14.12313 -5.8957 0.0000
## Birch-Beech 1.78983 -2.32391 5.9036 0.9788
## Cherry-Beech -2.02857 -6.14230 2.0852 0.9382
## Chestnut-Beech -0.08257 -4.19631 4.0312 1.0000
## Holmoak-Beech -13.76726 -17.88100 -9.6535 0.0000
## Hornbeam-Beech -1.72094 -5.83468 2.3928 0.9853
## Lime-Beech 6.87093 2.75719 10.9847 0.0000
## Oak-Beech 7.97092 3.85718 12.0847 0.0000
## Pine-Beech 2.01719 -2.09654 6.1309 0.9409
## Rowan-Beech -6.02870 -10.14244 -1.9150 0.0001
## Spruce-Beech -14.47485 -18.58859 -10.3611 0.0000
## Sycamore-Beech -9.51465 -13.62839 -5.4009 0.0000
## Willow-Beech -9.54730 -13.66104 -5.4336 0.0000
## Cherry-Birch -3.81839 -7.93213 0.2953 0.1011
## Chestnut-Birch -1.87240 -5.98614 2.2413 0.9682
## Holmoak-Birch -15.55709 -19.67082 -11.4433 0.0000
## Hornbeam-Birch -3.51077 -7.62451 0.6030 0.1925
## Lime-Birch 5.08110 0.96736 9.1948 0.0031
## Oak-Birch 6.18109 2.06735 10.2948 0.0001
## Pine-Birch 0.22737 -3.88637 4.3411 1.0000
## Rowan-Birch -7.81853 -11.93227 -3.7048 0.0000
## Spruce-Birch -16.26468 -20.37841 -12.1509 0.0000
## Sycamore-Birch -11.30448 -15.41822 -7.1907 0.0000
## Willow-Birch -11.33713 -15.45087 -7.2234 0.0000
## Chestnut-Cherry 1.94599 -2.16775 6.0597 0.9559
## Holmoak-Cherry -11.73869 -15.85243 -7.6250 0.0000
## Hornbeam-Cherry 0.30762 -3.80611 4.4214 1.0000
## Lime-Cherry 8.89949 4.78576 13.0132 0.0000
## Oak-Cherry 9.99949 5.88575 14.1132 0.0000
## Pine-Cherry 4.04576 -0.06798 8.1595 0.0592
## Rowan-Cherry -4.00014 -8.11387 0.1136 0.0662
## Spruce-Cherry -12.44628 -16.56002 -8.3325 0.0000
## Sycamore-Cherry -7.48609 -11.59982 -3.3723 0.0000
## Willow-Cherry -7.51874 -11.63247 -3.4050 0.0000
## Holmoak-Chestnut -13.68468 -17.79842 -9.5709 0.0000
## Hornbeam-Chestnut -1.63837 -5.75211 2.4754 0.9909
## Lime-Chestnut 6.95350 2.83977 11.0672 0.0000
## Oak-Chestnut 8.05349 3.93976 12.1672 0.0000
## Pine-Chestnut 2.09977 -2.01397 6.2135 0.9194
## Rowan-Chestnut -5.94613 -10.05986 -1.8324 0.0002
## Spruce-Chestnut -14.39227 -18.50601 -10.2785 0.0000
## Sycamore-Chestnut -9.43208 -13.54582 -5.3183 0.0000
## Willow-Chestnut -9.46473 -13.57846 -5.3510 0.0000
## Hornbeam-Holmoak 12.04632 7.93258 16.1601 0.0000
## Lime-Holmoak 20.63819 16.52445 24.7519 0.0000
## Oak-Holmoak 21.73818 17.62444 25.8519 0.0000
## Pine-Holmoak 15.78445 11.67072 19.8982 0.0000
## Rowan-Holmoak 7.73856 3.62482 11.8523 0.0000
## Spruce-Holmoak -0.70759 -4.82133 3.4061 1.0000
## Sycamore-Holmoak 4.25261 0.13887 8.3663 0.0350
## Willow-Holmoak 4.21996 0.10622 8.3337 0.0381
## Lime-Hornbeam 8.59187 4.47813 12.7056 0.0000
## Oak-Hornbeam 9.69186 5.57813 13.8056 0.0000
## Pine-Hornbeam 3.73814 -0.37560 7.8519 0.1207
## Rowan-Hornbeam -4.30776 -8.42150 -0.1940 0.0302
## Spruce-Hornbeam -12.75391 -16.86764 -8.6402 0.0000
## Sycamore-Hornbeam -7.79371 -11.90745 -3.6800 0.0000
## Willow-Hornbeam -7.82636 -11.94010 -3.7126 0.0000
## Oak-Lime 1.09999 -3.01375 5.2137 0.9999
## Pine-Lime -4.85373 -8.96747 -0.7400 0.0063
## Rowan-Lime -12.89963 -17.01337 -8.7859 0.0000
## Spruce-Lime -21.34578 -25.45951 -17.2320 0.0000
## Sycamore-Lime -16.38558 -20.49932 -12.2718 0.0000
## Willow-Lime -16.41823 -20.53197 -12.3045 0.0000
## Pine-Oak -5.95373 -10.06746 -1.8400 0.0001
## Rowan-Oak -13.99962 -18.11336 -9.8859 0.0000
## Spruce-Oak -22.44577 -26.55951 -18.3320 0.0000
## Sycamore-Oak -17.48557 -21.59931 -13.3718 0.0000
## Willow-Oak -17.51822 -21.63196 -13.4045 0.0000
## Rowan-Pine -8.04589 -12.15963 -3.9322 0.0000
## Spruce-Pine -16.49204 -20.60578 -12.3783 0.0000
## Sycamore-Pine -11.53185 -15.64558 -7.4181 0.0000
## Willow-Pine -11.56450 -15.67823 -7.4508 0.0000
## Spruce-Rowan -8.44615 -12.55988 -4.3324 0.0000
## Sycamore-Rowan -3.48595 -7.59969 0.6278 0.2019
## Willow-Rowan -3.51860 -7.63234 0.5951 0.1896
## Sycamore-Spruce 4.96020 0.84646 9.0739 0.0045
## Willow-Spruce 4.92755 0.81381 9.0413 0.0050
## Willow-Sycamore -0.03265 -4.14639 4.0811 1.0000
plot(TukeyHSD(model))
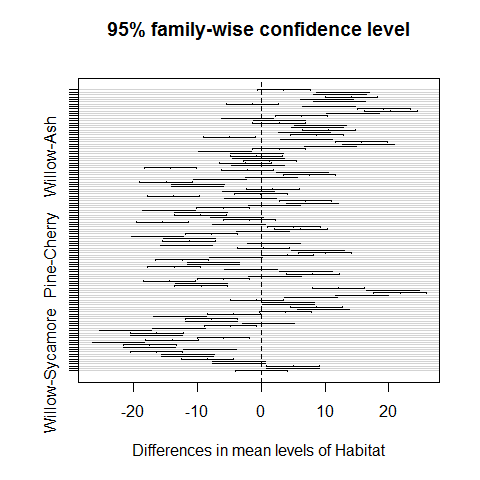 Habitats on opposite sides of the dotted line and not overlapping it are significantly different one from another.
Habitats on opposite sides of the dotted line and not overlapping it are significantly different one from another.
Alternatively, it is possible to use the pairwise.t.test function in which we specify the response variable, and then the categorical explanatory variable containing the factorlevels we want to compare, separated by a comma:
pairwise.t.test(Fugus.yield,Habitat)
##
## Pairwise comparisons using t tests with pooled SD
##
## data: Fugus.yield and Habitat
##
## Alder Ash Aspen Beech Birch Cherry Chestnut Holmoak
## Ash 0.10011 - - - - - - -
## Aspen < 2e-16 6.3e-11 - - - - - -
## Beech < 2e-16 5.4e-10 1.00000 - - - - -
## Birch < 2e-16 1.2e-13 1.00000 1.00000 - - - -
## Cherry 4.7e-13 2.9e-06 0.87474 1.00000 0.04943 - - -
## Chestnut < 2e-16 7.8e-10 1.00000 1.00000 1.00000 1.00000 - -
## Holmoak 1.00000 0.00181 < 2e-16 < 2e-16 < 2e-16 3.9e-16 < 2e-16 -
## Hornbeam 1.1e-13 8.6e-07 1.00000 1.00000 0.10057 1.00000 1.00000 < 2e-16
## Lime < 2e-16 < 2e-16 1.1e-05 1.9e-06 0.00131 3.3e-10 1.4e-06 < 2e-16
## Oak < 2e-16 < 2e-16 1.4e-07 2.0e-08 2.7e-05 1.9e-12 1.5e-08 < 2e-16
## Pine < 2e-16 3.9e-14 1.00000 1.00000 1.00000 0.02757 1.00000 < 2e-16
## Rowan 1.8e-05 0.51826 8.5e-06 4.7e-05 3.9e-08 0.03053 6.2e-05 5.3e-08
## Spruce 1.00000 0.00016 < 2e-16 < 2e-16 < 2e-16 < 2e-16 < 2e-16 1.00000
## Sycamore 0.50084 1.00000 2.1e-12 1.9e-11 3.3e-15 1.5e-07 2.7e-11 0.01586
## Willow 0.51826 1.00000 1.9e-12 1.6e-11 2.8e-15 1.4e-07 2.4e-11 0.01702
## Hornbeam Lime Oak Pine Rowan Spruce Sycamore
## Ash - - - - - - -
## Aspen - - - - - - -
## Beech - - - - - - -
## Birch - - - - - - -
## Cherry - - - - - - -
## Chestnut - - - - - - -
## Holmoak - - - - - - -
## Hornbeam - - - - - - -
## Lime 1.3e-09 - - - - - -
## Oak 8.4e-12 1.00000 - - - - -
## Pine 0.05975 0.00253 6.1e-05 - - - -
## Rowan 0.01380 < 2e-16 < 2e-16 1.5e-08 - - -
## Spruce < 2e-16 < 2e-16 < 2e-16 < 2e-16 2.5e-09 - -
## Sycamore 4.2e-08 < 2e-16 < 2e-16 1.1e-15 0.10218 0.00187 -
## Willow 3.8e-08 < 2e-16 < 2e-16 9.3e-16 0.10057 0.00203 1.00000
##
## P value adjustment method: holm
The default method of adjustment of the p values is holm, but other adjustment methods include hochberg, hommel, bonferroni, BH, BY, fdr and none.
Factorial ANOVA
A factorial experiment has two or more factors, each with two or more levels, plus replication for each combination of factors levels. This means that we can investigate statistical interactions, in which the response to one factor depends on the level of another factor.
Our example comes from a farm-scale trial of animal diets. There are two factors: diet and supplement. Diet is a factor with three levels: barley, oats and wheat. Supplement is a factor with four levels: agrimore, control, supergain and supersupp. The response variable is weight gain after 6 weeks.
weight <- read.delim("Z:/GitHub/RMarkdowns - Stat with R/6_ANOVA/growth.txt")
Tools for data exploration
# attach(weight)
# replications(gain ~ diet * supplement, data=weight) #this shows that the design is fully balanced
#
# #try to unbalance the design
# replications(gain ~ diet * supplement,data=weight[1:40,])
#
# boxplot(gain ~ diet + supplement, ylab="Weight gain" )
#
# #interaction plot
# interaction.plot(diet, supplement, #x.factor=diet, trace.factor=supplement,response=gain
# gain, fun=mean, type="b", legend=T, #type b plots either lines and points
# ylab="Weight gain", main="Interaction Plot",
# pch=c(1,19))
#
# coplot(gain ~ diet | supplement, data = weight, panel = panel.smooth,
# xlab = "Weight gain data: diet vs gain, given type of supplement")
#
# plot.design(gain ~ diet * supplement, data=ToothGrowth)
# barplot(tapply(gain,list(diet,supplement),mean),
# beside=T,ylim=c(0,30),col=c("orange","yellow","cornsilk")) #(note the use of beside=T to get the bars in adjacent clusters rather than vertical stacks
#
# labs <- c("Barley","Oats","Wheat")
# legend(3,29,labs,fill= c("orange","yellow","cornsilk"))
# #legend(locator(1),labs,fill= c("orange","yellow","cornsilk"))
#
# tapply(gain,list(diet,supplement),mean)
We use either lm and aov to run a a factorial analysis of variance
# model <- aov(gain~diet*supplement)
# summary(model)
No hint about a role of the interaction.
Model simplification
# model <- aov(gain~diet+supplement)
#
# par(mfrow=c(1,2))
# boxplot(gain~diet); boxplot(gain~supplement)
# par(mfrow=c(1,1))
#
# summary(model)
The disadvantage of the ANOVA table is that it does not show us the effect sizes. Let’s check the summary.lm
# summary.lm(model)
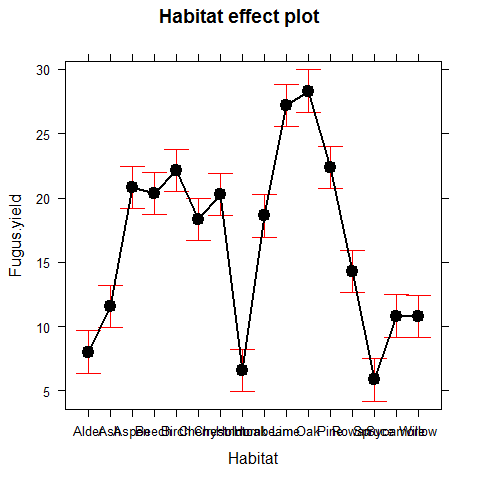
library(effects) # explore the library(effects) and make sure you know which model classes you can deal with
## Warning: package 'effects' was built under R version 3.0.3
plot(allEffects(model))
Pseudoreplication: Nested designs and split plots
The model-fitting functions aov, lme and lmer have the ease to deal with complicated error structures, and it is important to be able to recognize such error structures, and hence avoid the pitfalls of pseudoreplication.
There are two general cases:
1) split-plot analysis, when designed experiments have different treatments applied to plots of different sizes
2) nested sampling, when repeated measurements are taken from the same individual, or observational studies are conducted at several different spatial scales (mostly random effects);
Split-plot experiments
In a split-plot experiment, different treatments are applied to plots of different sizes. Each different plot size is associated with its own error variance, so instead of having one error variance (as in all the ANOVA tables up to this point), we have as many error terms as there are different plot sizes. The analysis is presented as a series of component ANOVA tables, one for each plot size, in a hierarchy from the largest plot size with the lowest replication at the top, down to the smallest plot size with the greatest replication at the bottom.
The following example refers to a designed field experiment on crop yield with three treatments: irrigation (with two levels, irrigated or not), sowing density (with three levels, low, medium and high), and fertilizer application (with three levels, low, medium and high).
yields <- read.delim("Z:/GitHub/RMarkdowns - Stat with R/6_ANOVA/splityield.txt")
attach(yields)
names(yields)
## [1] "yield" "block" "irrigation" "density" "fertilizer"
head(yields)
## yield block irrigation density fertilizer
## 1 90 A control low N
## 2 95 A control low P
## 3 107 A control low NP
## 4 92 A control medium N
## 5 89 A control medium P
## 6 92 A control medium NP
summary(yields)
## yield block irrigation density fertilizer
## Min. : 60.0 A:18 control :36 high :24 N :24
## 1st Qu.: 86.0 B:18 irrigated:36 low :24 NP:24
## Median : 95.0 C:18 medium:24 P :24
## Mean : 99.7 D:18
## 3rd Qu.:114.0
## Max. :136.0
level 1 -> 4 blocks level 2 -> irrigation (each half of a block) level 3 -> seed-sowing densities (in each 1/3 of irrigation splits) level 4-> each density plot was split into 3, and one of the fertilizer nutrient treatment N, P, N and P together (only one of the )
model =aov(yield~irrigation*density*fertilizer+Error(block/irrigation/density)) #Note that the smallest plot size, fertilizer, does not need to appear in the Error term
summary(model)
##
## Error: block
## Df Sum Sq Mean Sq F value Pr(>F)
## Residuals 3 194 64.8
##
## Error: block:irrigation
## Df Sum Sq Mean Sq F value Pr(>F)
## irrigation 1 8278 8278 17.6 0.025 *
## Residuals 3 1412 471
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
##
## Error: block:irrigation:density
## Df Sum Sq Mean Sq F value Pr(>F)
## density 2 1758 879 3.78 0.053 .
## irrigation:density 2 2747 1374 5.91 0.016 *
## Residuals 12 2788 232
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
##
## Error: Within
## Df Sum Sq Mean Sq F value Pr(>F)
## fertilizer 2 1977 989 11.45 0.00014 ***
## irrigation:fertilizer 2 953 477 5.52 0.00811 **
## density:fertilizer 4 305 76 0.88 0.48405
## irrigation:density:fertilizer 4 235 59 0.68 0.61067
## Residuals 36 3109 86
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
It is possible to see the four ANOVA tables, one for each plot size: blocks are the biggest plots, half blocks get the irrigation treatment, one third of each half block gets a sowing density treatment, and one third of a sowing density treatment gets each fertilizer treatment. Note that the non-significant main effect for density (p = 0.053) does not mean that density is unimportant, because density appears in a significant interaction with irrigation (the density terms cancel out, when averaged over the two irrigation treatments; see below).
The best way to understand the two significant interaction terms is to plot them using interaction.plot:
interaction.plot(fertilizer,irrigation,yield)
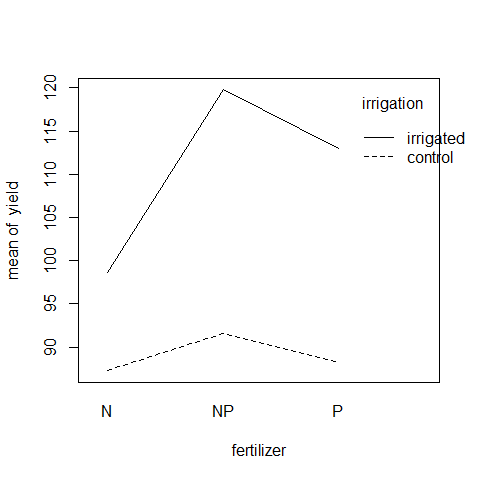
Irrigation increases yield proportionately more on the N-fertilized plots than on the P-fertilized plots. The irrigation-density interaction is more complicated:
interaction.plot(density,irrigation,yield)
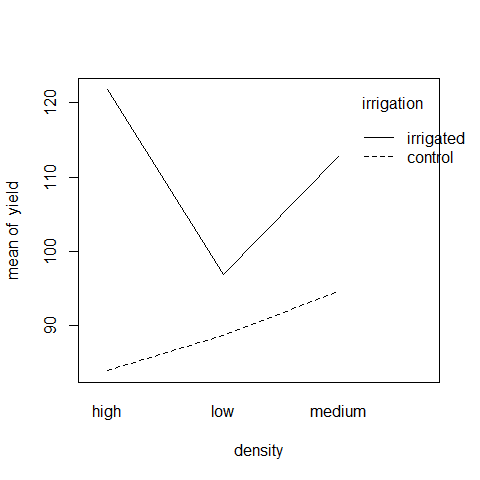 On the irrigated plots, yield is lowest on the low-density plots, but on control plots yield is lowest on the high-density plots.
On the irrigated plots, yield is lowest on the low-density plots, but on control plots yield is lowest on the high-density plots.
Effect sizes in ANOVA: aov or lm?
The difference between lm and aov is mainly in the output form: The summary table with aov is in the traditional form for the analysis of variance, with one row for each categorical variable and each interaction term. On the other hand, the summary table for lm produces one row per estimated parameter (i.e. one row for each factor level and one row for each interaction level). If there are multiple error terms (spatial pseudoreplication) then an aov approach must be used because lm does not support the Error term.
Here is a three-way analysis of variance fittdtted firstrst using aov first and lm after:
daphnia <- read.delim("Z:/GitHub/RMarkdowns - Stat with R/6_ANOVA/daphnia.txt")
attach(daphnia)
names(daphnia)
## [1] "Growth.rate" "Water" "Detergent" "Daphnia"
model1 <- aov(Growth.rate~Water*Detergent*Daphnia)
summary(model1)
## Df Sum Sq Mean Sq F value Pr(>F)
## Water 1 2.0 1.99 2.85 0.09784 .
## Detergent 3 2.2 0.74 1.06 0.37548
## Daphnia 2 39.2 19.59 28.13 8.2e-09 ***
## Water:Detergent 3 0.2 0.06 0.08 0.96861
## Water:Daphnia 2 13.7 6.87 9.86 0.00026 ***
## Detergent:Daphnia 6 20.6 3.43 4.93 0.00053 ***
## Water:Detergent:Daphnia 6 5.8 0.97 1.40 0.23432
## Residuals 48 33.4 0.70
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
All three factors are likely to stay in the model because every of them is involved in at least one significant interaction. We must not be misled by the apparently non-significant main effect for detergent. The three-way interaction is clearly non-significant and can be deleted (p = 0.234).
Here is the output from the same analysis using the linear model function:
model2 <- lm(Growth.rate~Water*Detergent*Daphnia)
summary(model2)
##
## Call:
## lm(formula = Growth.rate ~ Water * Detergent * Daphnia)
##
## Residuals:
## Min 1Q Median 3Q Max
## -1.4882 -0.5440 0.0239 0.3560 1.5250
##
## Coefficients:
## Estimate Std. Error t value
## (Intercept) 2.8113 0.4818 5.83
## WaterWear -0.1581 0.6814 -0.23
## DetergentBrandB -0.0354 0.6814 -0.05
## DetergentBrandC 0.4763 0.6814 0.70
## DetergentBrandD -0.2141 0.6814 -0.31
## DaphniaClone2 0.4964 0.6814 0.73
## DaphniaClone3 2.0553 0.6814 3.02
## WaterWear:DetergentBrandB 0.4646 0.9636 0.48
## WaterWear:DetergentBrandC -0.2743 0.9636 -0.28
## WaterWear:DetergentBrandD 0.2173 0.9636 0.23
## WaterWear:DaphniaClone2 1.3808 0.9636 1.43
## WaterWear:DaphniaClone3 0.4316 0.9636 0.45
## DetergentBrandB:DaphniaClone2 0.9189 0.9636 0.95
## DetergentBrandC:DaphniaClone2 -0.1634 0.9636 -0.17
## DetergentBrandD:DaphniaClone2 1.0121 0.9636 1.05
## DetergentBrandB:DaphniaClone3 -0.0649 0.9636 -0.07
## DetergentBrandC:DaphniaClone3 -0.8079 0.9636 -0.84
## DetergentBrandD:DaphniaClone3 -1.2867 0.9636 -1.34
## WaterWear:DetergentBrandB:DaphniaClone2 -1.2638 1.3628 -0.93
## WaterWear:DetergentBrandC:DaphniaClone2 1.3561 1.3628 1.00
## WaterWear:DetergentBrandD:DaphniaClone2 0.7762 1.3628 0.57
## WaterWear:DetergentBrandB:DaphniaClone3 -0.8744 1.3628 -0.64
## WaterWear:DetergentBrandC:DaphniaClone3 -1.0302 1.3628 -0.76
## WaterWear:DetergentBrandD:DaphniaClone3 -1.5540 1.3628 -1.14
## Pr(>|t|)
## (Intercept) 4.5e-07 ***
## WaterWear 0.8175
## DetergentBrandB 0.9588
## DetergentBrandC 0.4879
## DetergentBrandD 0.7547
## DaphniaClone2 0.4699
## DaphniaClone3 0.0041 **
## WaterWear:DetergentBrandB 0.6319
## WaterWear:DetergentBrandC 0.7771
## WaterWear:DetergentBrandD 0.8226
## WaterWear:DaphniaClone2 0.1584
## WaterWear:DaphniaClone3 0.6563
## DetergentBrandB:DaphniaClone2 0.3451
## DetergentBrandC:DaphniaClone2 0.8661
## DetergentBrandD:DaphniaClone2 0.2988
## DetergentBrandB:DaphniaClone3 0.9466
## DetergentBrandC:DaphniaClone3 0.4060
## DetergentBrandD:DaphniaClone3 0.1881
## WaterWear:DetergentBrandB:DaphniaClone2 0.3584
## WaterWear:DetergentBrandC:DaphniaClone2 0.3247
## WaterWear:DetergentBrandD:DaphniaClone2 0.5716
## WaterWear:DetergentBrandB:DaphniaClone3 0.5241
## WaterWear:DetergentBrandC:DaphniaClone3 0.4534
## WaterWear:DetergentBrandD:DaphniaClone3 0.2598
## ---
## Signif. codes: 0 '***' 0.001 '**' 0.01 '*' 0.05 '.' 0.1 ' ' 1
##
## Residual standard error: 0.835 on 48 degrees of freedom
## Multiple R-squared: 0.715, Adjusted R-squared: 0.578
## F-statistic: 5.23 on 23 and 48 DF, p-value: 7.02e-07
Note that the two significant interactions from the aov table do not show up in the summary.lm table (Water- Daphnia and Detergent-Daphnia). This is because summary.lm shows treatment contrasts, comparing everything to the Intercept, rather than orthogonal contrasts. In the aov table, the p values are ‘on deletion’ p values, which is a big advantage.
In complicated designed experiments, it is easier to summarize the effect sizes with plot.design and model.tables functions.
For main effects:
plot.design(Growth.rate~Water*Detergent*Daphnia)
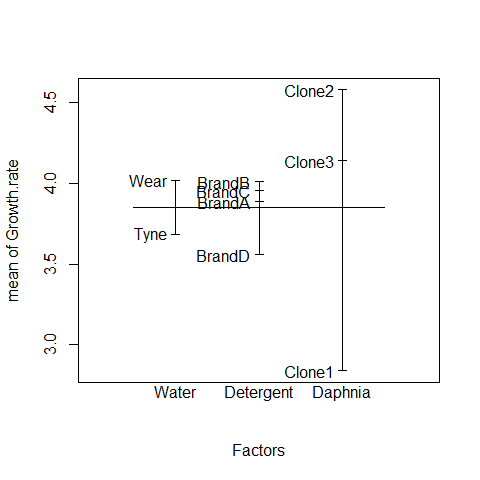
This simple graphical device provides a very clear summary of the three sets of main effects. It is no ideal, however, at illustrating the interactions. The model.tables function takes the name of the fitted model object as its firrst argument, and then it is possible to specify whether we want the standard errors (as we typically would):
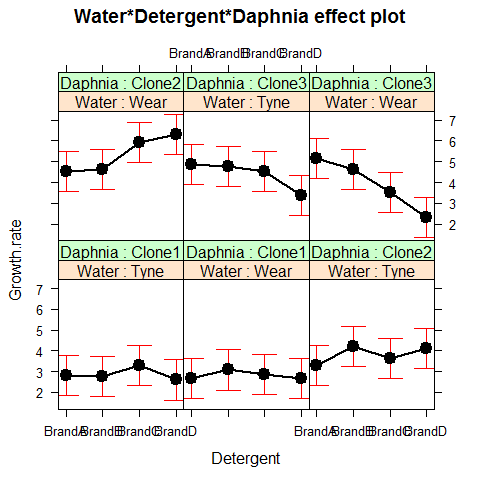
Attractive plots of effect sizes can be obtained using the effects library.
library(effects)
plot(allEffects(model2))
ANOVA for repeated measures
This time, we have repeated measures of the same animal, sample sampling unit etc, producing a set of dependent populations. As long as we have only categorical predictors and balanced designs, it is possible to deal with it using repeated measures ANOVAs
Examples: measures of the same individual before and after the treatment (“client of a psycological clinic”). It is inappropriate to just compare the before and after observations as if they were independent, because they are not. The “after” observations are usually dependent upon the “before” observations and the effect of order. That is, a client who was very anxious at the initial occasion of measurement is likely to be less anxious at subsequent occasions, and because of the selection of clients on the basis of their disturbed psychological state and the change that state over time, our clients (who tended to be more disturbed than usual when they were recruited) will be likely to get somewhat better even if we just tell them to come back in six weeks.
# the aov for repeated measures will take the general form: summary(aov(response~contrasts+Error(ID),mydata))
The NON-PARAMETRIC version of the ANOVA for repeated measures is the Friedman test
The next level includes nested and crossed random effects, categorical and/or numerical predictors, unbalanced sample designs, spatial and temporal autocorrelations —» MIXED-EFFECTS models